Digital Intelligence Transformation, Standards Lead | Keyuan Pharma participated in the formulation of the "Guidelines for Digital Transformation Construction of Pharmaceutical Enterprises"

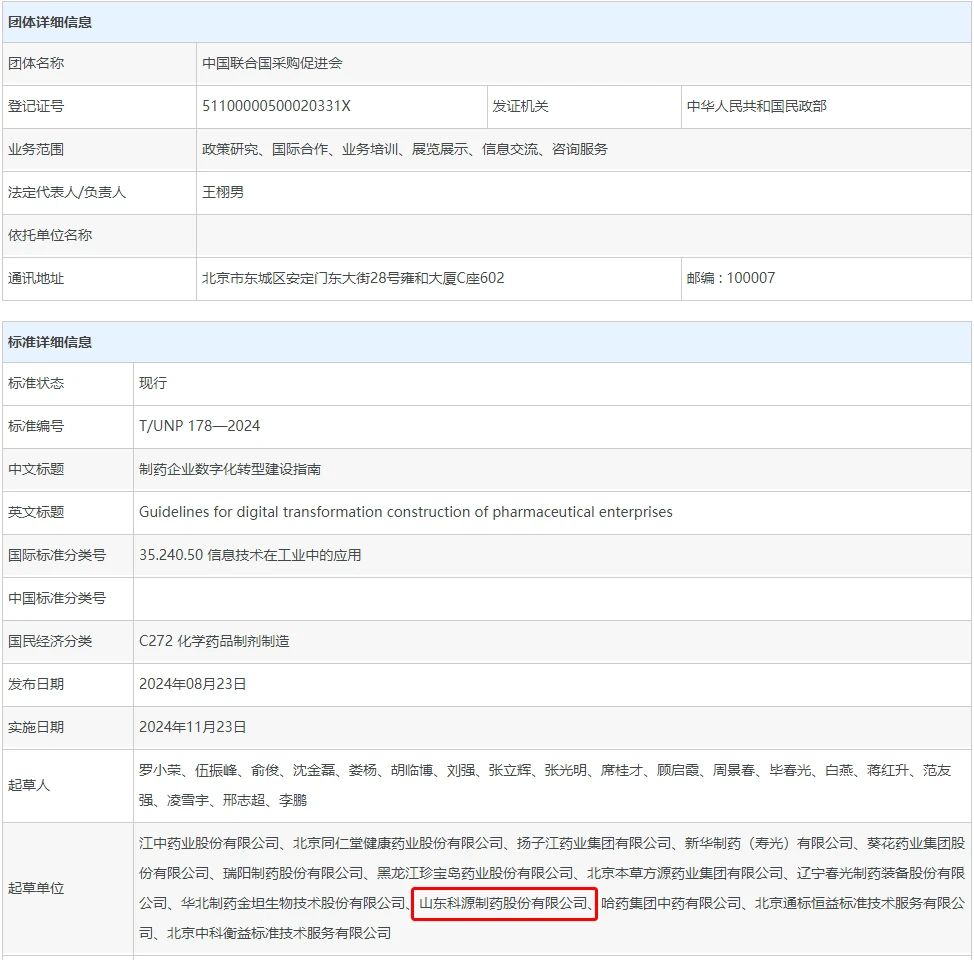
Recently, the China United Nations Procurement Promotion Association issued the group standard T/UNP 178-2024 "Guidelines for Digital Transformation Construction of Pharmaceutical Enterprises", marking an important step for Chinese pharmaceutical companies in digital transformation. Shandong Keyuan Pharmaceutical Co., Ltd. (stock code: 301281.SZ), as one of the important drafting units, actively participated in the formulation of this standard.
The "Guidelines for Digital Transformation Construction of Pharmaceutical Enterprises" mainly covers transformation planning, resource support, operation implementation, evaluation and inspection, and improvement and enhancement. By clarifying the goals, paths and measures of digital transformation, it helps enterprises establish a scientific digital transformation system, realize the reengineering and optimization of business processes, and enhance the core competitiveness and market response speed of enterprises.
It is reported that the group standard of "Guidelines for Digital Transformation Construction of Pharmaceutical Enterprises" was officially released on August 23, 2024, and will be officially implemented on November 23, 2024. The standard follows the principles of openness, fairness, transparency, consensus and promotion of trade and exchanges, and aims to guide pharmaceutical companies to smoothly promote digital transformation and improve overall operational efficiency and market competitiveness.
As a leading company in the domestic chemical API segment, Keyuan Pharma has always led high-quality development with technological innovation and industrial upgrading. This participation in the formulation of the "Guidelines for Digital Transformation of Pharmaceutical Enterprises" not only reflects Keyuan Pharma's profound accumulation in digital transformation, but also demonstrates leading position and influence in the industry.
At a time when the global health industry is undergoing profound changes, the digital transformation of the health industry is not just an upgrade, but also a revolution and a leap forward, which will surely become the core driving force for the development of the industry in the future.
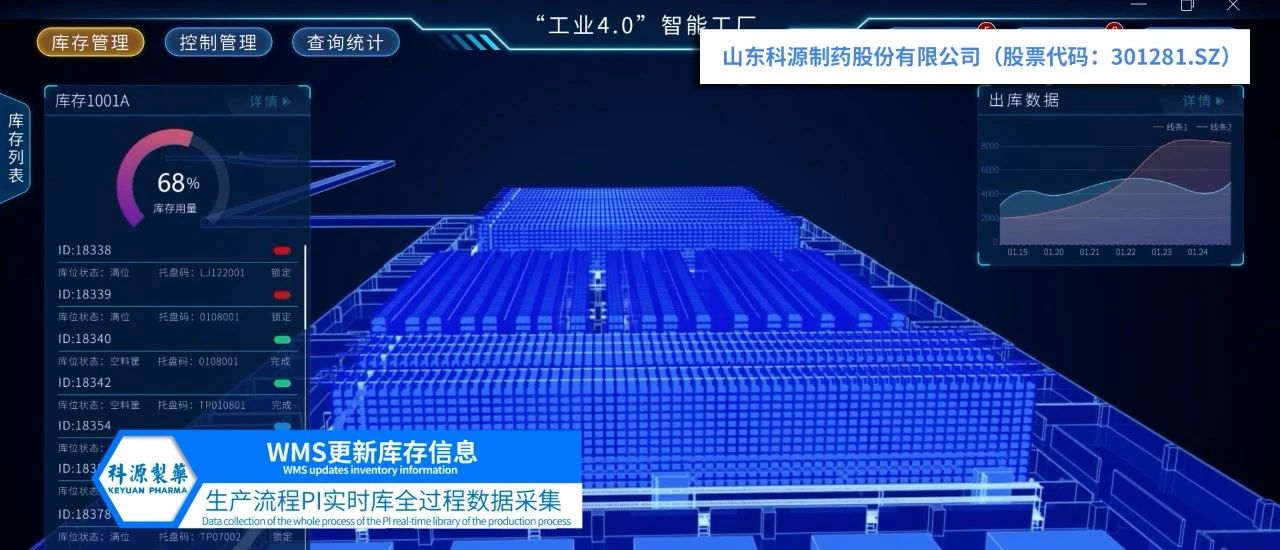
In the tide of digital medicine transformation, Keyuan Pharma, with high industry sensitivity and innovation capabilities, actively responds to Chinese policies, takes smart factories, digital workshops, and unmanned production lines as development direction, realizes the integrated application of enterprise resource planning, product life cycle management, and supply chain management systems, and accelerates the digital and intelligent transformation and upgrading of enterprises.
With outstanding innovation capabilities and significant digital and intelligent transformation results, Keyuan Pharma is honored to be listed in the list of "2024 Shandong Province Chemical Industry Intelligent Transformation Benchmark Enterprise"!

During the formulation of the "Guidelines for Digital Transformation of Pharmaceutical Enterprises", Keyuan Pharma combined its own practical experience and put forward many valuable opinions and suggestions, which were eventually adopted and incorporated into the standard, providing important reference and guidance for the digital transformation of pharmaceutical enterprises.

Keyuan Pharma indicated that participating in the formulation of this standard is a concrete action to actively respond to Chinese policies and promote the development of the industry. In the future, Keyuan Pharma will continue to uphold the core concept of "Healthy China, Committed with Heart", continue to increase investment and efforts in digital transformation, and set an example and contribute to the transformation and upgrading and high-quality development of the pharmaceutical industry.

